Journal of Surgery and Insights
Research Article
Impact of Immediate Breast Reconstruction after Mastectomy on the short and Long-Term Outcomes of Patients Receiving Neoadjuvant Chemotherapy
Nogi H1*, Shioya H1, Toriumi Y1, Tomita S2, Nagasaki E3 and Takeyama H1
1Department of Breast and Endocrine Surgery, The Jikei University School of Medicine, Japan
2Department of Plastic Surgery, The Jikei University School of Medicine,Japan
3Department of Medical Oncology and Hematology, The Jikei University School of Medicine, Japan
*Corresponding author: Hiroko Nogi, Department of Breast and Endocrine Surgery, The Jikei University School of Medicine, Japan.
Citation: Nogi H, Shioya H, Toriumi Y, Tomita S, Nagasaki E, et al. (2020) Impact of Immediate Breast Reconstruction after Mastectomy on the short and Long-Term Outcomes of Patients Receiving Neoadjuvant Chemotherapy. J Surg Insights: JSI-100032
Received date: 25 July, 2020; Accepted date: 30 July, 2020; Published date: 06 August, 2020
Abstract
Objectives: In breast cancer patients receiving neoadjuvant chemotherapy (NAC), immediate breast reconstruction (IBR) after mastectomy remains controversial. We retrospectively investigated whether IBR influenced the oncological outcomes and complications of patients receiving NAC.
Methods: Between August 2005 and December 2018, 568 breast cancer cases received NAC at our institute, of whom 57, 216, and 295 patients underwent IBR after mastectomy (IBR), mastectomy alone (M-alone), and breast conserving surgery (BCS), respectively. The IBR group and the non-IBR groups were compared in terms of clinic pathological characteristics and prognosis.
Results: IBR-related complication occurred in one patient with flap necrosis. After a median of 83.7 months, loco regional recurrence was found in one patient (1.8%) in the IBR group, 18 patients (8.3%) in the M-alone group, and 16 (5.4%) patients in the BCS group. The 5-year loco regional recurrence-free survival among those groups was 98.3%, 92.4% and 95.5% (p=0.06), and the 5-year breast cancer-related death-free survival was 98.2%, 90.3%, and 98.2%, respectively (p<0.001).
Conclusions: IBR after NAC had no association with worse prognosis in this study. IBR after NAC can be performed safely under careful technique and could be considered as a strategy preferred for patients with local advanced breast cancer.
Keywords: Breast cancer; Breast reconstruction; Neoadjuvant chemotherapy; Prognosis
Abbreviations
BCRDFS : Breast cancer related death-free survival
BCS : Breast-conserving surgery
DDFS : Distant disease-free survival
HER2 : Human epidermal growth-factor receptor-2
IBR : Immediate breast reconstruction
LLFS : Recurrence-free survival
M-alone : Mastectomy alone
NAC : Neoadjuvant chemotherapy
PMRT : Post mastectomy radiation therapy
SBI : Silicone breast implants
Introduction
Immediate breast reconstruction (IBR) after mastectomy is a treatment option for patients with early-stage breast cancer when breast-conserving surgery (BCS) is infeasible, or the diagnostic age is young [1-3]. Neoadjuvant chemotherapy (NAC) reduces the cancer burden in both cases with breast lymph nodes and those with axillar lymph nodes before surgery and the residual tumor status can indicate prognosis [4-7]. Though certain studies reported that postoperative complications increased among patients receiving IBR after NAC, and the appropriate adjuvant therapy was delayed [8,9]many studies showed no significant increase in complications and no worse prognosis in patients with IBR after NAC [10-12].Therefore, whether IBR after NAC should be performed remains unclear. This study aimed to retrospectively investigate whether IBR after NAC influenced patients’ short- and long-term outcomes.
Materials and methods
Patients: We retrospectively reviewed the records of 568 consecutive breast cancer patients who received anthracycline and taxane as NAC at the Jikei University Hospital between August 2005 and December 2018. Patients undergoing IBR, mastectomy alone, and BCS were compared in terms of age, clinicopathological characteristics i.e. clinical tumor size and clinical lymph node status before NAC, estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 (HER2) expression, pathological tumor size, pathological lymph node status, (lymph vascular infiltration,) and survival data. This study was in accordance with the Helsinki Declaration of 1975, (as revised in 1983) and approved by the Ethics Committee of the Jikei University School of Medicine, and patient consent was obtained.
Treatment intervention:Reconstruction was chosen based on patient preference and the consultation with a plastic surgeon at our institution, as no guideline for IBR after NAC was currently available. The multidisciplinary panel of experts at our institution, which included oncologists, radiologists, surgeons, and reconstructive surgeons, discussed the PMRT schedule for patients with IBR in order to develop consensus. Tissue expander was replaced with silicone breast implant (SBI) within 2 to 3 months, and PMRT was started within 4 to 6 weeks after surgery. In other surgical procedures, such as direct-to-implant breast reconstruction, BCS, and mastectomy alone, radiotherapy was started 6-8 weeks later.
All patients received NAC with four cycles of epirubicin (100 mg/m2), 5-fluorouracil (500 mg/m2), and cyclophosphamide (500 mg/m2), followed by four cycles of docetaxel (75 mg/m2), or twelve cycles of paclitaxel (80 mg/m2). If patients were HER2-positive, trastuzumab was administered concurrently with taxane and adjuvant trastuzumab for a 1-year duration. A 5-to 10-year adjuvant hormonal therapy was used if patients were hormone receptor-positive.
The patients with BCS received the whole breast radiotherapy, and the regional lymph node radiotherapy was used for patients with ≥4 positive nodes and considered in patients with 1-3 positive nodes. The decision making for PMRT followed the standard guidelines such as National Comprehensive Cancer Network guidelines which recommend using PMRT for patients with initial tumors ≥5 cm, those with ≥4 positive nodes, and considered in those with 1-3 positive nodes. PMRT is not a contraindication for patients receiving IBR; however, they were informed about the risk of complications, for example capsular contracture. Infection, dehiscence, hematoma, flap or nipple necrosis, capsule contracture, or implant failure were defined as post-operative complications. Clinical tumor size was based on the largest size identified on ultrasound, or contrast-magnetic resonance imaging. Clinical lymph nodes were evaluated using ultrasound and biopsy.Statistical analysis
For the statistical analysis, patients were divided into three groups, namely the IBR group, which consisted of patients undergoing IBR after mastectomy; the M-alone group, including patients with mastectomy alone; and the BCS group, including patients undergoing BCS. Clinicopathological data were tabulated for each group. Difference in the distribution of subject characteristics among three groups were evaluated by the Pearson chisquare and Kruskal-Wallis test. The primary endpoints for statistical analysis included the locoregional recurrence-free survival (LLFS), distant disease-free survival (DDFS), and breast cancer related death-free survival (BCRDFS). Loco regional recurrence was defined as the recurrence of tumor within the ipsilateral chest wall, including skin, subcutaneous tissue, and pectoralis muscle, or regional lymph nodes (in the ipsilateral axillary, supraclavicular, internal mammary, or infra-clavicular areas). Distant metastasis was defined as the recurrence of tumor in distant organs or lymph nodes (i.e. beyond the above areas). The Kaplan–Meier method was used to generate survival curves and the cumulative incidence of events. Survival was calculated from the date of surgery to the date of the event or latest follow-up. The log-rank test was used to assess differences in the Kaplan-Meier curves. The Cox regression model was used to identify potential prognostic and predictive indicators. All significant test was two-sided, p values ≤0.05 were considered to be significant. All analyses were performed using the Stata statistical software (StataSE 10; Stata Corp LP, College Station, TX). The secondary endpoint was the incidence of complications related to IBR.
Results
In this study, we analyzed data from the records of 568 patients, including 57, 216, and 295 undergoing IBR, mastectomy alone, and BCS, respectively. Table 1 presents characteristics of patients and tumors. The median age was 46 (range; 29-73) years old in the IBR group, 56 (range; 24-83) in the M-alone group and 56 (range; 29-83) in the BCS. The IBR group was significantly younger than the other two groups (p<0.001); the BCS group had a smaller tumor size and a higher proportion of node-negative disease before NAC than the other two groups (p<0.001); while the M-alone group had a significantly larger clinical and yield pathological tumor size than the other two groups (p<0.001 for both clinical and yield pathological tumor size). The BCS group had a significantly higher proportion of negative lymph node infiltration and negative pathological node than the other two groups (p=0.02).
The complication associated with IBR was seen in one patient with transverse rectus abdominis muscle
flap necrosis. Other complications such as infection, dehiscence, hematoma, nipple necrosis, capsule contracture, or implant failure were not observed.
After a median of 83.7 (range; 5.4-170.2) months, 1 patient (1.8%) in the IBR group, 18 patients (8.3%) in the M-alone group, and 16 (5.4%) patients in the BCS group showed loco regional recurrence. Figure 1 shows the cumulative LLFS of the three patient groups. The 5-year LLFS was 98.3% (95%CI; 88.2 - 99.8) in the IBR group, 95.5% (95%CI; 92.3-97.4) in the BCS group, and 92.4% (95% CI; 86.1-95.6) in the M-alone group.
The LLFS in the M-alone group was worse than in the other groups (p=0.06). Multivariate analysis revealed that the large yield pathological tumor size and the HER2 positive expression were associated with locoregional recurrence (Table 3).
Figure 2depicts the cumulative DDFS. The 5-year DDFS was 89.5% (95% CI; 76.4-95.5) in the IBRgroup, 95.8% (95% CI; 92.5-97.7) in the BCS group, and 83.6% (95% CI; 77.6-88.1) in the M-alone group. The DDFS in the BCS group was higher than that in the other groups (p<0.001). Multivariate analysis revealed that the large clinical and yield pathological tumor size, positive clinical node, and positive vascular infiltration were associated with distant metastasis (Table 3).
Figure 3shows the cumulative BCRDFS. The 5-year BCRDFS survival was significantly lower in the
M-alone group (90.3%, 95% CI: 85.1-93.7%, p<0.001) than the IBR group (98.2%, 95% CI: 88.0-99.8%) and the BCS group (98.2%, 95% CI: 95.7-99.2%). Multivariate analysis revealed that the large clinical and yield pathological tumor size, positive pathological node, PgR negativity, HER2 positivity, and positive vascular infiltration were associated with a higher breast cancer-related mortality rate (Table 3). IBR had no impact on the three types of survival.
Discussion
We demonstrated the oncological safety of IBR after NAC. Our data showed that worse prognosis was associated with advanced stage before and after NAC and biology such as HER2 positivity or PgR negativity.
Breast cancer is the most frequent malignant disease among women worldwide [13,14], including Japan [15]. Recent advances in chemotherapy and anti-HER2 targeted agents have led to the increased use of NAC. Initially, NAC was used to convert locally advanced tumors from inoperable to operable, or downsize tumors to allow for BCS [4-7].
Currently, it is used to assess the in vivo chemo sensitivity and biology of breast tumors and the long-term outcomes of breast cancer patients based on tumor responses [16,17]. Patients who fail to obtain the pathological complete response are candidates for adjuvant chemotherapies such as TDM1 or capecitabine [18,19]. Thanks to these advances, clinicians have increasingly used NAC for breast cancer. With patients’ better response to NAC, clinicians need to consider decisions on the type of operation, the timing of breast reconstruction, as well as the axillary management and appropriate use of radiotherapy. Considering the increasing importance and frequency of NAC in breast cancer treatment, it is imperative to assess its impacts on IBR. IBR is an option for any women receiving mastectomy. It restores body image, improves vitality, femininity, and sexuality, and has positive impacts on patients’ psychological well-being and quality of life [1-3].
However, locally advanced breast cancer patients who had previously undergone a modified radical mastectomy with delayed breast reconstruction and those with reduced tumor burden thanks to NAC may be candidates for IBR. The main problem of IBR which reported increased postoperative complications would be the need to delay the adjuvant therapy, such as PMRT, anti-HER2 agents and capecitabine, which leads increase in the rate of local recurrence of disease and decreases in the life expectancy.
Implant-based IBR is most frequently used today [20]. Common complications which arise from tissue expander/implant-based IBR include hematoma, seroma, infection, extrusion, and tissue necrosis. It was reported that the rate of complications after placement of a tissue expander/implant ranged between 16% and 49%[10-12]. The increased rate of complications was associated with NAC, PMRT, and patient-related factors such as smoking and elevated body mass index, less surgeon reconstructive experience and postoperative infection. To avoid these complications, surgeons should keep the surgical field and prosthesis clean, confirm the hemostasis, and use antibiotics postoperatively. Patient education and support plays an important role in discouraging patients from smoking and encouraging them to do weight loss exercises while using NAC. In this present study, most patients with IBR had normal BMI and no smoking habit; hence, fewer numbers of complications were observed.
A meta-analysis demonstrated that the use of IBR after NAC did not affect overall and disease-free survival of breast cancer patients or the local recurrence rate [21,22]. Our data also showed that loco regional recurrence of breast cancer and overall survival were not affected by IBR after NAC. However, reported the high rate of locoregional recurrence in patients with ER-negative disease [23]. In our study, locoregional recurrence occurred in only one patient with IBR who had ER-negative disease.
PMRT improves local control, disease-free survival, and overall survival among women with stage II/III breast cancer [24-26]. PMRT was used for those with ≥4 positive lymph nodes for several decades. However, after a meta-analysis demonstrated an improvement in disease-free and overall survival among women with even one positive lymph node, the therapy is now more frequently recommended as a breast cancer treatment option for those with 1-3 positive lymph nodes [27,28]. In contrast, IBR followed by PMRT tends not to be recommended due to higher complication rates and poor aesthetic outcomes. PMRT has been reported to have an association with a higher rate of implant loss (9.1-25.0%), compared to non-radiated group [29,30]. In a large prospective study, compared long-term outcomes between two groups of patients (one group receiving breast reconstruction with PMRT to the tissue expander and the other receiving radiation to the implant). The study demonstrated that the 6-year predicted failure rate among patients who received radiation to the tissue expander before it was exchanged for a permanent implant was twice as much as that among those receiving radiation after placement of the permanent implant [31]. Therefore, the final implant is recommended to be irradiated to minimize reconstructive failure. In our study, no implant failure, flap necrosis and capsular contracture were observed.
Our findings demonstrated that IBR after mastectomy did not affect breast cancer recurrence and patient survival, neither did it increase the frequency of local recurrence. The present study has several limitations that need to be addressed. This is a retrospective study conducted at a single institution. In addition, the relatively small number of patients in the IBR group limited the statistical power of the analysis. The IBR group included the more patients who had good response to NAC than M-alone group. The follow-up period is relatively short to evaluate the oncological safety of PMRT for silicone breast implants. All limitations and risks of bias are inherent in the retrospective design, thereby hindering the generalization of the study results to other populations. The strength of this study lies in its long follow-up periods for evaluating oncological safety of patients receiving IBR after NAC.
Conclusion
IBR after NAC can be performed safely. IBR after NAC could be considered as a preferred strategy for patients with local advanced breast cancer.
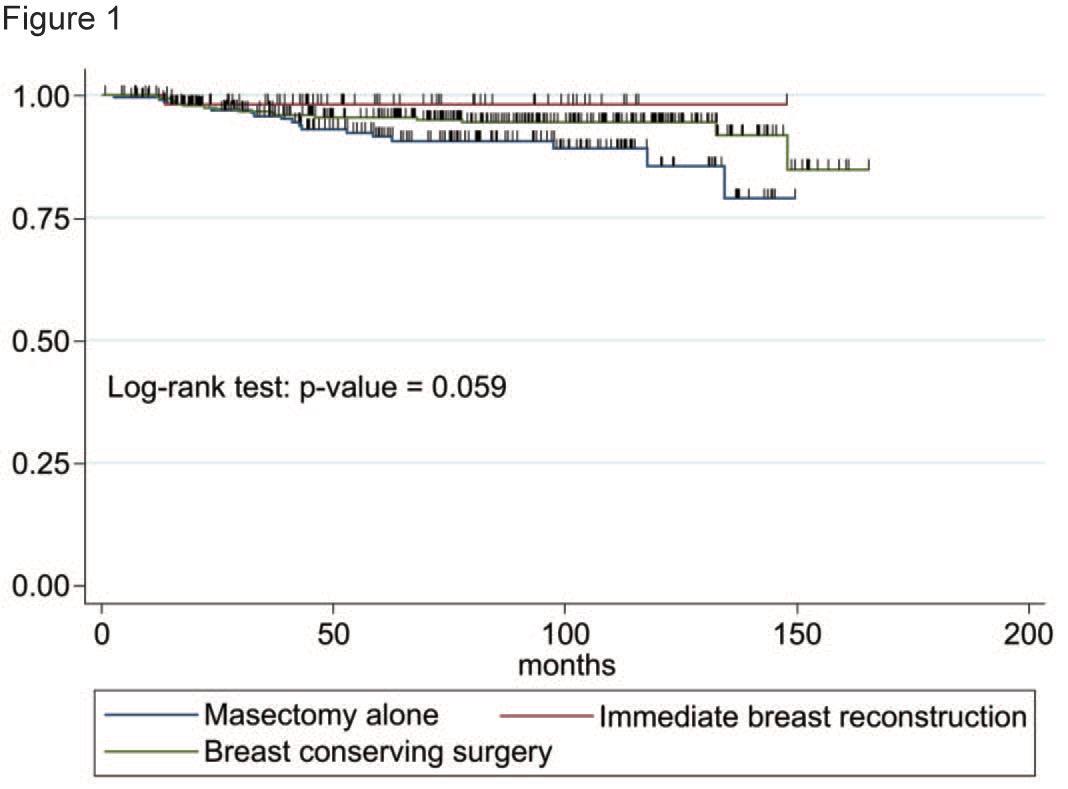
Figure 1:Locoregional recurrence-free survival of three groups.
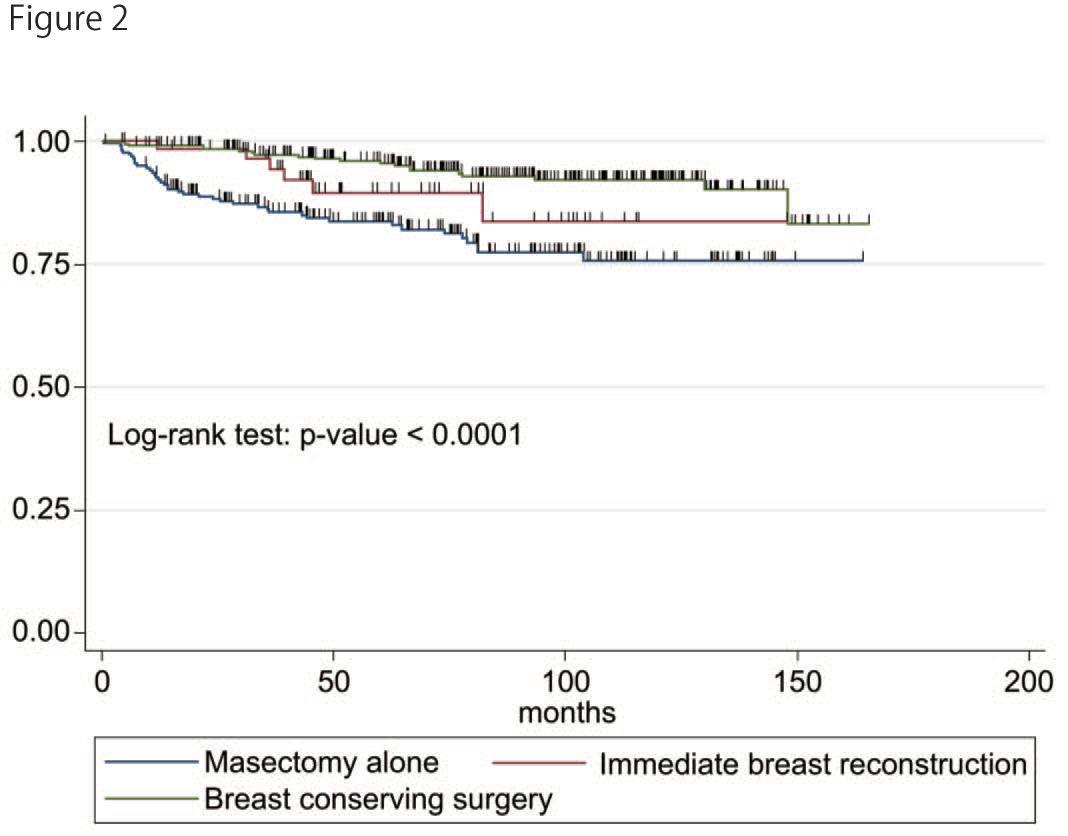
Figure 2: Distant disease-free survival of three groups.
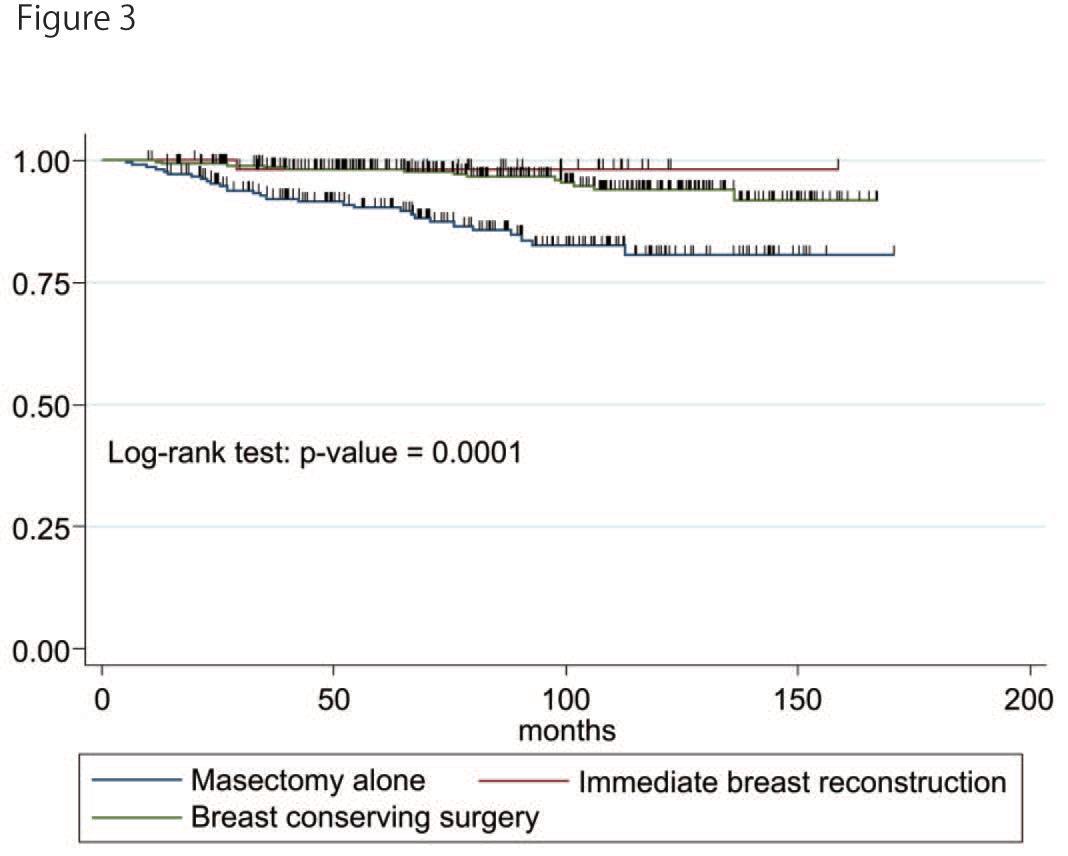
Figure 3: Breast cancer related death-free survival of three groups.
|
- |
IBR (n=57) |
M-alone (n=216) |
BCS (n=295) |
p value |
|
|
N (%) |
N (%) |
N (%) |
|
||
|
Age (years) |
Median |
46 |
56 |
54 |
<0.001 |
|
|
range |
29-73 |
29-83 |
24-78 |
|
|
Clinical T |
0/1 |
21(36.8) |
26(21.0) |
66 (22.4) |
<0.001 |
|
|
2 |
23 (40.4) |
104 (48.2) |
208 (70.5) |
|
|
|
3 |
7 (12.3) |
56 (25.9) |
10 (3.4) |
|
|
|
4 |
6 (10.5) |
30 (13.9) |
11 (3.7) |
|
|
Clinical N |
Negative |
23 (40.3) |
85 (39.4) |
180 (61.0) |
<0.001 |
|
Positive |
34 (59.7) |
131(60.6) |
125 (39.0) |
||
|
Estrogen |
Negative |
13 (22.8) |
84 (38.9) |
97 (32.9) |
0.06 |
|
Receptor |
Positive |
44 (77.2) |
132 (61.6) |
198 (67.1) |
|
|
Progesterone |
Negative |
22 (38.6) |
121 (56.0) |
152 (51.5) |
0.06 |
|
receptor |
Positive |
35 (61.4) |
95 (44.0) |
143 (48.5) |
|
|
HER2 |
Negative |
44 (77.2) |
147 (68.0) |
218 (73.9) |
0.23 |
|
Positive |
13 (22.8) |
69 (32.0) |
77 (26.1) |
||
|
ypT |
0 |
15 (26.3) |
40(18.5) |
72 (24.4) |
<0.01 |
|
1 |
24 (42.1) |
67 (31.0) |
128 (43.4) |
||
|
2 |
16 (28.1) |
57 (26.4) |
87 (29.5) |
||
|
3 |
2 (3.5) |
51 (23.6) |
8 (2.7) |
||
|
4 |
0 (0.0) |
1 (0.5) |
0 (0.0) |
||
|
ypN |
Negative |
34 (59.7) |
125 (57.9) |
205 (69.5) |
0.02 |
|
Positive |
23 (40.3) |
91 (42.1) |
90 (30.5) |
||
|
ly |
Negative |
50 (87.7) |
174 (80.6) |
264 (89.5) |
0.02 |
|
Positive |
7 (12.3) |
42 (19.4) |
31 (10.5) |
||
|
v |
Negative |
56 (98.3) |
205 (94.9) |
286 (96.9) |
0.34 |
|
Positive |
1 (1.2) |
11 (5.1) |
9 (3.1) |
||
|
BCS: Breast conserving surgery; HER2: Human epidermal growth factor receptor-2; IBR: Immediate breast reconstruction; ly: Lymph node infiltration; M-alone: Mastectomy alone; N: Nodes; T: Tumor; v: vascular infiltration; ypN: Yield pathological nodes; ypT: Yield pathological tumor. |
|||||
Table 1: Patient and tumor characteristics.
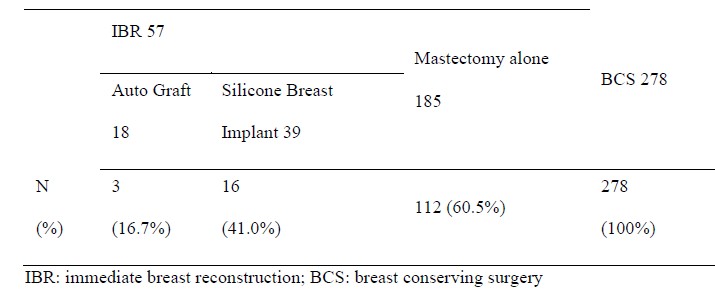
Table 2: Number and percentage of patients receiving radiotherapy.
|
- |
Locoregional recurrence |
Distant disease |
Death |
|||
|
HR |
95% CI |
HR |
95% CI |
HR |
95% CI |
|
|
IBR |
0.35 |
0.05-2.58 |
1.03 |
0.43-2.42 |
0.27 |
0.36-2.02 |
|
ER |
0.7 |
0.28-1.75 |
0.94 |
0.46-1.94 |
0.91 |
0.43-1.94 |
|
PgR |
0.62 |
0.25-1.52 |
0.79 |
0.43-1.45 |
0.33 |
0.16-0.72 |
|
HER2 |
2.56 |
1.24-5.28 |
1.04 |
0.55-1.98 |
2.77 |
1.41-5.42 |
|
cT |
1.03 |
0.66-1.63 |
1.45 |
1.06-2.00 |
1.63 |
1.09-2.43 |
|
cN |
1.59 |
0.75-3.51 |
2.02 |
1.13-3.62 |
1.26 |
0.63-2.52 |
|
ypT |
1.63 |
1.08-2.46 |
1.85 |
1.34-2.57 |
1.64 |
1.13-2.39 |
|
ypN |
1.3 |
0.59-2.86 |
1.35 |
0.77-2.37 |
2.79 |
1.32-5.92 |
|
ly |
2.61 |
0.73-4.48 |
1.56 |
0.85-2.89 |
1.96 |
0.93-4.14 |
|
v |
- |
- |
2.43 |
1.06-5.58 |
3.94 |
1.38-11.24 |
|
IBR: Immediate breast reconstruction; ER: Estrogen receptor; PgR: Progesterone receptor; HER2: Human epidermal growth factor receptor-2; ypT: Yield pathological tumor; ypN: Yield pathological nodes; T: Tumor; N: Nodes; ly: Lymph node infiltration; V: Vascular infiltration |
||||||
Table 3: Multivariate survival analysis.
Citation: Nogi H, Shioya H, Toriumi Y, Tomita S, Nagasaki E, et al. (2020) Impact of Immediate Breast Reconstruction after Mastectomy on the short and Long-Term Outcomes of Patients Receiving Neoadjuvant Chemotherapy. J Surg Insights: JSI-100032